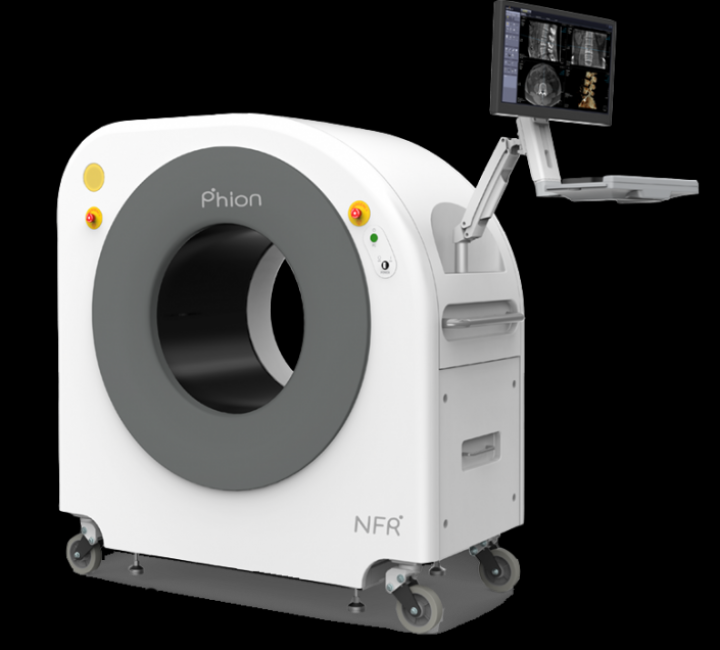
We are pleased to announce the signing of an overseas distributor contract for the the sales of NanoFocusRay Mobile CT Phion 2.0.
Please refer to press release:
http://www.newsa.co.kr/news/articleView.html?idxno=253132
http://www.hrdnews.org/news/articleView.html?idxno=4849
Phion 2.0 is a portable CT that can be installed and operated anywhere, providing for quick examinations in the field that can relieve patients' anxety. Radiation dosage is also one-fourth that of a normal CT, reducing the patient's radiation exposure burden.
Phion 2.0 was originally developed for musculoskeletal and spinal imaging, but due to the COVID-19 pandemic, it was also certified by the South Korean Ministry of Food and Drug Safety (MFDS) on 11 March 2020 for the diagnosis of COVID-19 patients.
Please visit www.avatahealthcare.com for more information.